Plastic pollution is a colossal environmental problem, with millions of tons of it dumped into the ocean every year. And while many nations around the world have made an effort to step up their recycling game in recent years, there’s clearly more work to be done: When it comes to plastic water bottles in the United States alone, for instance, only about one-sixth to one-third of them actually end up being recycled.
A paper out Friday in the journal Science Advances describes a new recycling method aimed to help tackle the issue. The study demonstrates a relatively simple and efficient process that can be used to break down one of the most common types of plastic waste into usable fuel.
“As a chemist, we understand these polymers are extremely inert — they’re very stable — and if you don’t do anything, they can persist in the water or soil for many hundreds to a thousand years without breaking down,” said Zhibin Guan, a chemistry professor at the University of California at Irvine and one of the study’s senior authors. “We thought we should work on something to address this issue.”
The study focuses on a type of plastic known as polyethylene, a material that’s used in many products, including bottles or jugs to plastic bags. The authors of the new study note that it’s the highest-volume type of plastic in the world — some sources indicate that up to 100 million metric tons of it are produced each year.
Plastics, in general, are typically made from petroleum, natural gas or sometimes other organic materials in the first place — so it’s possible to turn them back into fuels or other useful hydrocarbons. The problem with polyethylene is that it’s an extremely stable plastic, meaning it doesn’t react easily with other chemicals. This makes it difficult to break down.
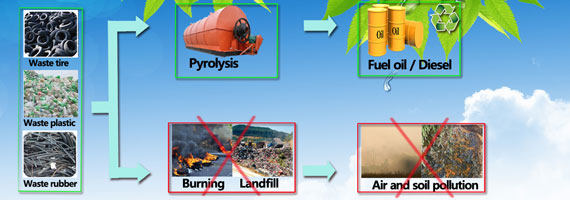
Nevertheless, some processes do already exist to make it happen — and some companies are even trying to put them to commercial use. But they often require extreme temperatures to work and aren’t always that efficient, Guan said.
A process called pyrolysis, for instance, essentially heats up plastic at high temperatures — often over 700 degrees Fahrenheit — causing it to vaporize. Then, it can be condensed back down into a liquid form and refined into a fuel. Alternatively, some scientists have used highly reactive chemicals called radicals to break down polyethylene under less extreme conditions.
But both strategies tend to produce a mixture of products at the end with different chemical formulas, and it’s hard to control for a specific desired end result, Guan said. So he and colleagues from both the University of California at Irvine and the Shanghai Institute of Organic Chemistry at the Chinese Academy of Sciences collaborated to develop a method that would work under mild conditions and would easily produce a desired product — in this case, oils and waxes fit for commercial use.
Such research on simpler methods for the breakdown of plastics is important for the future of plastic recycling, Kevin McDonnell, an expert in energy technology at the University College Dublin, noted by email. “Given the low value of commercial fuel oils, low-cost techniques that can produce a usable product are critical to the successful recovery of plastic waste,” he wrote.
The new method builds on a technique originally developed by other research groups from Rutgers University and the University of North Carolina at Chapel Hill for use in building up alkanes rather than breaking them down, Guan said. He and his team were able to tweak the method for their own purposes.
The technique uses specific types of hydrocarbons, known as alkanes, to help break up the long chemical chains in polyethylenes through a process known as cross alkane metathesis, or CAM. Polyethylene itself is essentially composed of long alkanes, the researchers noted — the CAM process helps to scramble or rearrange them chemically, ultimately converting them into different products.
The materials used to make the method work can be acquired conveniently from other industrial processes, Guan pointed out. For instance, the researchers found that a substance known as petroleum ether, which can be generated as a side product during oil refinery, can be used in their method — and it’s typically inexpensive and easy to come by, he said.
The method ultimately breaks down polyethylene into both liquid fuels and waxes — and the researchers found that it works on a variety of different types of polyethylenes. Furthermore, the researchers found that the method is also successful when applied to commercial plastic waste, which typically include extra chemical additives, such as antioxidants, that they’d worried might have affected their process.
The researchers hope that their method will eventually become a commercial success, one that will help make a dent in the huge amounts of plastic waste currently occupying landfills and floating in the seas all over the world.
“At this moment, we are still working on a few issues to make it more efficient,” Guan said. For instance, the researchers would like to increase the amount of fuel they can get out of the process and also conduct more research on the ability to recycle the materials used to conduct the process.
Of course, there’s another environmental consideration here — the fact that fuel produced from plastic is still fuel, meaning it still releases greenhouse gas emissions when burned. Some research has suggested that fuel from plastic waste via pyrolysis may produce fewer emissions than standard gasoline — but, nevertheless, it’s not exactly a “clean” technology.
On the other hand, fossil fuels are likely to remain an integral part of the global energy mix for decades to come, even as alternative energy sources expand. And because plastic production doesn’t appear to be going anywhere anytime soon either, conversations about how to deal with the waste in innovative ways are still necessary.
“The global community lifestyle is based on carbon — be that the energy we use, the food we eat, clothes we wear, technology we use — so we are not going to switch off the carbon pipeline easily,” McDonnell pointed out. “Plastic plays a key role in that carbon chain — therefore the more we can recover and recycle the carbon in the plastics we use, the lower the burden we place on the planet from the use of that plastic.”
Correction: A previous version of this article stated that the method described builds on a technique originally designed for use in building up polymers. In fact, the technique was developed for use in building up alkanes. The article also previously stated that petroleum ether can be generated as a side product during oil extraction. It is a side product of oil refinery.
Article source: https://www.washingtonpost.com/news/energy-environment/wp/2016/06/17/one-way-to-tackle-our-huge-plastic-pollution-problem-turn-it-into-fuel/